Asymmetric hydroamination catalyzed by a new chiral zirconium system: reaction scope and mechanism†
Abstract
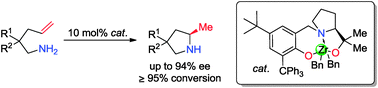
A new class of chiral zirconium complexes supported by chiral tridentate [O−NO−]-type of ligands derived from amino acids were synthesized and structurally characterized. They catalyzed asymmetric hydroamination/cyclization of primary aminoalkenes to give five- and six-membered N-heterocyclic amines with up to 94% ee.

 Please wait while we load your content...
Please wait while we load your content...