Transition-metal-free persulfuration to construct unsymmetrical disulfides and mechanistic study of the sulfur redox process†
Abstract
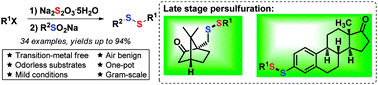
A sulfur redox process has been developed between sulfinate and thiosulfate, which efficiently affords diverse unsymmetrical disulfides and provides a new method to modify pharmaceuticals and natural products without requiring an extra oxidant or reductant. Gram-scale investigation further demonstrates the practicality and application potential of this process. Isolated key intermediates and a series of control experiments afford an unusual process, which reveals the mechanism of comproportionation and the transition-metal-free sulfur redox process.

 Please wait while we load your content...
Please wait while we load your content...