Metal controlled regioselectivity in the cyclometallation of 2-(1-naphthyl)-pyridine†
Abstract
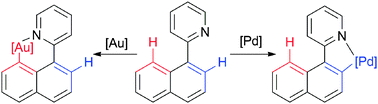
Cyclometallation of 2-(1-naphthyl)-pyridine is described. While cyclopalladation results in a five-membered metallacycle, cycloauration displays a completely orthogonal regioselectivity, resulting in the six-membered ring analogue. Bromination of the gold metallacycle results in the new C–H functionalisation product 2-(8-bromonaphth-1-yl)pyridine.

 Please wait while we load your content...
Please wait while we load your content...