Facile synthesis of RGD peptide-modified iron oxide nanoparticles with ultrahigh relaxivity for targeted MR imaging of tumors†
Abstract
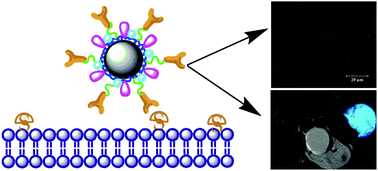
We report the facile synthesis of arginine-glycine-aspartic acid (RGD) peptide-targeted iron oxide (Fe3O4) nanoparticles (NPs) with ultrahigh relaxivity for in vivo tumor magnetic resonance (MR) imaging. In this study, stable polyethyleneimine (PEI)-coated Fe3O4 NPs were first prepared by a mild reduction route. The formed aminated Fe3O4 NPs with PEI coating were sequentially conjugated with fluorescein isothiocyanate (FI) and polyethylene glycol (PEG)-RGD segment, followed by acetylation of the remaining PEI surface amines. The thus-formed Fe3O4@PEI·NHAc-FI-PEG-RGD NPs were characterized via different techniques. We show that the multifunctional RGD-targeted Fe3O4 NPs with a mean size of 9.1 nm are water-dispersible, colloidally stable, and hemocompatible and cytocompatible in the given concentration range. With the displayed ultrahigh r2 relaxivity (550.04 mM−1 s−1) and RGD-mediated targeting specificity to αvβ3 integrin-overexpressing cancer cells as confirmed by flow cytometry and confocal microscopy, the developed multifunctional Fe3O4@PEI·NHAc-FI-PEG-RGD NPs are able to be used as a highly efficient nanoprobe for targeted MR imaging of αvβ3 integrin-overexpressing cancer cells in vitro and the xenografted tumor model in vivo. Given the versatile PEI amine-enabled conjugation chemistry, the developed PEI-coated Fe3O4 NPs may be functionalized with other biological ligands or drugs for various biomedical applications, in particular, the diagnosis and therapy of different types of cancer.

 Please wait while we load your content...
Please wait while we load your content...