A new potentiometric sensor for the determination of ketamine hydrochloride in ampoules and urine
Abstract
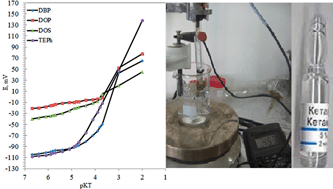
Ketamine drug in urine and pharmaceutical preparations was determined by a new chemically modified carbon paste electrode (CMCPE) based on an ion-exchanger of ketamine hydrochloride with sodium tetraphenylborate (KT-TPB) as a chemical modifier. The best performance was exhibited by the electrode having a paste containing 0.5 wt% ion-exchanger (KT-TPB), 54.3 wt% graphite, 45.0 wt% tris(2-ethylhexyl) phosphate (TEPh) and 0.2 wt% sodium tetraphenylborate (Na-TPB). The prepared electrode showed a Nernstian slope of 58.9 ± 0.3 mV per decade for ketamine ions in the concentration range of 9.0 × 10−6 M to 1.0 × 10−2 M with a limit of detection of 7.3 × 10−6 M. The electrode has a short and stable response time of 8 s and good reproducibility, and it can be used in a pH range of 3.7–6.6. The selective coefficients were determined in relation to several inorganic and organic ions, sugars and some common drug excipients. Ketamine is determined successfully in ampoule and urine using the standard addition and the calibration curve methods.

 Please wait while we load your content...
Please wait while we load your content...