A differential protein solubility approach for the depletion of highly abundant proteins in plasma using ammonium sulfate†
Abstract
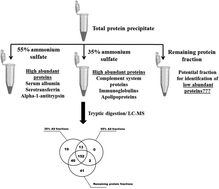
Depletion of highly abundant proteins is an approved step in blood plasma analysis by mass spectrometry (MS). In this study, we explored a precipitation and differential protein solubility approach as a fractionation strategy for abundant protein removal from plasma. Total proteins from plasma were precipitated with 90% saturated ammonium sulfate, followed by differential solubilization in 55% and 35% saturated ammonium sulfate solutions. Using a four hour liquid chromatography (LC) gradient and an LTQ-Orbitrap XL mass spectrometer, a total of 167 and 224 proteins were identified from the 55% and 35% ammonium sulfate fractions, whereas 235 proteins were found in the remaining protein fractions with at least two unique peptides. SDS-PAGE and exclusive total spectrum counts from LC-MS/MS analyses clearly showed that majority of the abundant plasma proteins were solubilized in 55% and 35% ammonium sulfate solutions, indicating that the remaining protein fraction is of potential interest for identification of less abundant plasma proteins. Serum albumin, serotransferrin, alpha-1-antitrypsin and transthyretin were the abundant proteins that were highly enriched in 55% ammonium sulfate fractions. Immunoglobulins, complement system proteins, and apolipoproteins were among other abundant plasma proteins that were enriched in 35% ammonium sulfate fractions. In the remaining protein fractions a total of 40 unique proteins were identified of which, 32 proteins were identified with at least 10 exclusive spectrum counts. According to PeptideAtlas, 9 of these 32 proteins were estimated to be present at low μg ml−1 (0.12–1.9 μg ml−1) concentrations in the plasma, and 17 at low ng ml−1 (0.1–55 ng ml−1) range.

 Please wait while we load your content...
Please wait while we load your content...