Solvent-induced structural transitions of lysozyme in an electrospray ionization source†
Abstract
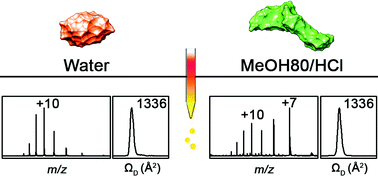
The structural characterization of proteins using electrospray ionization mass spectrometry (ESI-MS) has become an important method for understanding protein structural dynamics. The correlation between the structures of proteins in solution and gas phase needs to be understood for the application of ESI-MS to protein structural studies. Hen egg white lysozyme (Lyz) is a small protein with a stable compact structure in solution. Although it was known that denatured Lyz in solution undergoes compaction during transfer into the gas phase via ESI, detailed characterization of the process was not available. In the present study, we show that the organic cosolvent, which denatures Lyz in solution, induces the collapse of the extended Lyz structure into compact structures during ESI. This process is further facilitated by the presence of acids, whose conjugate bases can interact with Lyz to reduce its charge state and the electrostatic repulsion between its charged residues (Analyst, 2015, 140, 661–669). Exposure of ESI droplets to acid and solvent vapors confirms that the overall process most probably occurs in the charged droplets from ESI. This study provides a detailed understanding of the possible influence of the solvent environment on protein structure during transfer into the gas phase.

 Please wait while we load your content...
Please wait while we load your content...