Azide and trans-cyclooctene dUTPs: incorporation into DNA probes and fluorescent click-labelling†
Abstract
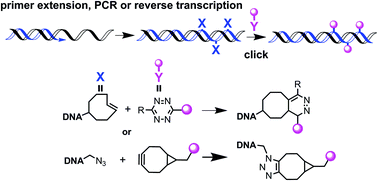
5-Azidomethyl dUTP and two 5-trans-cyclooctene dUTPs with different linkers between the TCO and the uracil base have been incorporated into DNA by primer extension, reverse-transcription and PCR amplification. For azidomethyl dUTP the PCR reaction was successful even when the modified dUTP was not supplemented with dTTP. In one case 335 azidomethyl dU residues were incorporated into the 523 base pair amplicon using this methodology. 5-Azidomethyl dUTP was found to be a better substrate for DNA polymerases than the trans-cyclooctene dUTPs. However, the inverse electron demand Diels–Alder reaction between cyclooctene DNA and a tetrazine Cy3-dye was more efficient than the strain-promoted reaction between azide DNA and a bicyclo [6.1.0] non-4-yne Cy3 dye.
- This article is part of the themed collection: Analytical Sciences in the UK

 Please wait while we load your content...
Please wait while we load your content...