Novel organic solvents for electrochemistry at the liquid/liquid interface
Abstract
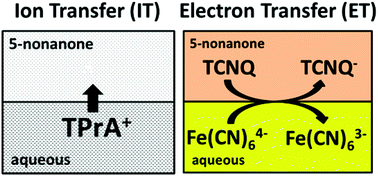
Two-phase voltammetry has been carried out using a reverse cell configuration, i.e. with the lower density organic solvent on the top of the aqueous solution in the cell, where the organic solvents contain either nitrile or ketone functional groups. The transfer of tetraphenylarsonium TPAs+, tetrabutylammonium TBA+, tetrapropylammonium TPrA+, and perchlorate CIO4− ions across these liquid/liquid interfaces has been observed. The standard Gibbs energies of ion partition from water to di-n-butyl ketone (5-nonanone) were calculated and compared with the previously reported 2-heptanone/water interface. Ion transfer (IT) and electron transfer (ET) were also investigated at the 5-nonanone/water interface. ET was exemplified using the ferri/ferrocyanide redox couple as the aqueous phase couple and the 7,7,8,8-tetracyanoquinodimethane (TCNQ) as the organic species.
- This article is part of the themed collection: Analytical Sciences in the UK

 Please wait while we load your content...
Please wait while we load your content...