Binding studies and anion-selective electrodes with neutral isophthalamide-based receptors†
Abstract
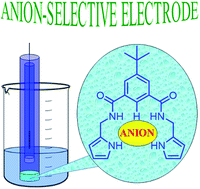
Two acyclic isophthalamide-based hosts have been synthesised and their anion binding properties have been evaluated by 1H-NMR titrations. Different binding modes have been detected for the series of tested anions. The attachment of aminomethylpyrrole groups resulted in an improved binding selectivity. Additionally, the receptors have been incorporated as ionophores in plasticised polymeric membrane-based anion-selective electrodes. The potentiometric studies were in agreement with the NMR experiments and revealed a good sensing ability, considering the structural simplicity of the receptors and their interactions purely based on hydrogen bonding. These preliminary experiments have revealed an interesting selectivity towards highly hydrophilic anions such as fluoride and sulfate. Moreover, a particularly low detection limit (9 × 10−7 M) has been determined for the fluoride anion.

 Please wait while we load your content...
Please wait while we load your content...