Experimental correlation between thermal hysteresis activity and the distance between antifreeze proteins on an ice surface†
Abstract
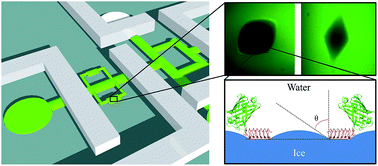
Antifreeze proteins (AFPs) aid the survival of cold-adapted organisms by inhibiting the growth of ice crystals in the organism. The binding of AFPs to ice separates the melting point from the freezing point of the ice crystal (thermal hysteresis, TH). Although AFPs were discovered more than 40 years ago, the mechanism by which they inhibit ice growth remains unclear. The distance between surface-bound AFPs is thought to correlate directly with the TH activity; however, this correlation has never been experimentally established. A novel microfluidics system was used here to obtain ice crystals covered with GFP-tagged AFPs in an AFP-free solution. This method permits calculation of the surface density of bound AFPs. Fluorescence intensity analysis revealed that the distance between ∼3 nm-long AFPs on the ice surface was 7–35 nm, depending on the AFP solution concentration and time of its exposure to ice. A direct correlation between these distances and the measured TH activity was found for a representative insect AFP, but not for a typical fish AFP. Insect AFPs accumulate over multiple ice crystal planes, especially the basal plane. Fish AFPs, which cannot bind to the basal plane, change the shape of the crystal to minimize the basal plane area. Thus, we postulate that the surface density of fish AFPs on the prism plane is not directly indicative of the TH activity, which ends when ice grows out of the basal plane and is a function of the basal plane area. These results significantly contribute to our understanding of the AFP mechanism and will be helpful in applying these proteins in different fields.


 Please wait while we load your content...
Please wait while we load your content...