Pd(ii)-catalyzed asymmetric addition of arylboronic acids to cyclic N-sulfonyl ketimine esters and a DFT study of its mechanism†‡
Abstract
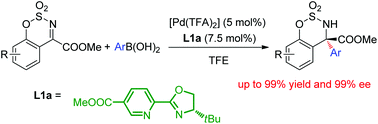
A palladium-catalyzed asymmetric arylation of cyclic N-sulfonyl ketimine esters is described. The desired products could be prepared with excellent yields (up to 99%) and enantioselectivities (up to 99% ee) under mild reaction conditions. Furthermore, a possible reaction mechanism was determined using DFT calculations.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2015 and Celebrating the 80th Birthday of Professor Ei-ichi Negishi

 Please wait while we load your content...
Please wait while we load your content...