Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates
Abstract
The transition metal-catalyzed allylic substitution of unactivated allylic substrates (allylic alcohols, allylic ethers and allylic amines) is rapidly becoming an important area of research. There are several advantages to using these substrates in allylic substitution reactions: the use of unactivated alcohols minimizes the production of waste by-products and reaction steps; and allylic ethers and allylic amines are useful substrates because of their stability and their presence in numerous biologically active compounds. Research in this field has therefore gained widespread attention for promoting the development of efficient and environmentally benign procedures for the formation of C–C, C–N and C–O bonds.
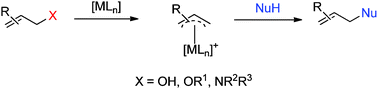

 Please wait while we load your content...
Please wait while we load your content...