Thermodynamic parameters of cation exchange in MOF-5 and MFU-4l†
Abstract
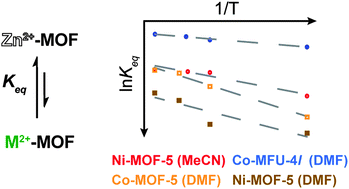
We present a method for approximating thermodynamic parameters 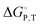 , ΔH, and ΔS for the cation exchange process in metal–organic frameworks, as exemplified by Ni2+ exchange into Zn4O(1,4-benzenedicarboxylate)3 (MOF-5) and Co2+ exchange into MOF-5 and Zn5Cl4(bis(1H-1,2,3-triazolo-[4,5-b],[4′,5′-i])dibenzo-[1,4]-dioxin)3 (MFU-4l). For these examples, we find that the cation exchange process is endergonic and that parameters such as solvent and cation identity impact the thermodynamics.
, ΔH, and ΔS for the cation exchange process in metal–organic frameworks, as exemplified by Ni2+ exchange into Zn4O(1,4-benzenedicarboxylate)3 (MOF-5) and Co2+ exchange into MOF-5 and Zn5Cl4(bis(1H-1,2,3-triazolo-[4,5-b],[4′,5′-i])dibenzo-[1,4]-dioxin)3 (MFU-4l). For these examples, we find that the cation exchange process is endergonic and that parameters such as solvent and cation identity impact the thermodynamics.

 Please wait while we load your content...
Please wait while we load your content...