Assessment of in vivo chronic toxicity of chitosan and its derivates used as oral insulin carriers
Abstract
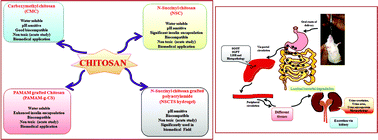
Considering public health protection, the carrier system for oral insulin must be safe. Hence, in the present study, the chronic oral toxicity of chitosan derivates was investigated in a mouse model. Oral administration of polymers did not cause any significant change in the behavioural pattern, body weight, and clinical symptoms of the treated mice. There were also no significant alterations in the biochemical parameters of blood serum and urine. Further, histopathological examination revealed an almost normal architecture, suggesting no significant adverse effects on the liver, kidney and intestine of the treated animals. An in vitro haemolysis assay proved that chitosan and its derivatives were blood compatible. Finally, intestinal luminal bacteria were able to biodegrade the polymers completely. Overall, the results suggested that the oral administration of the derivatives of chitosan in mice did not produce any significant toxicity in chronic treatment. Hence, these polymers could be utilized as safe devices for oral delivery of insulin and also other drugs.

 Please wait while we load your content...
Please wait while we load your content...