Bis-cationic ionic liquid crystals†
Abstract
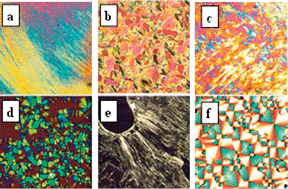
A series of bis-cationic imidazolium-based compounds are reported, where two imidazolium head groups are bridged by different types of spacers: a saturated C6 hydrocarbon spacer (type A), an ether bridge (type B), a benzylic spacer (type C) and a 2-butyne spacer (type D). The effect on mesophase formation of (1) the type of spacer, (2) the alkyl side chain length, and (3) the nature of the anion was investigated. The structural flexibility of the spacers seems to be responsible for the temperature range over which mesophases could be observed. Compared to types A, C, and D, the liquid crystalline state in type B is reached at much lower temperature and extends over a broader temperature range. As type B showed the best mesophase properties, we also combined the [B-C12] (bis(n-alkyl)-1,1-(oxydi-2,1-ethane-diyl)bis-imidazolium) cation with other anions such as ClO4−, BF4−, PF6− and the large, weakly coordinating anion bis(trifluoromethanesulfonyl)amide, NTf2−. For compounds [B-C12][BF4]2 and [B-C12][ClO4]2, with an anion of Td symmetry, a rare higher order smectic T phase could be observed, which transited into a smectic A phase during heating. Unexpectedly, mesophase formation was found for compound [B-C12][NTf2]2, which has not previously been observed for compounds with mono-cationic structures paired with this anion. In addition the rheological behavior of the bromide ILCs was investigated. The viscosities of all ILCs depend on the shear rate applied to the sample when the salt is in its mesophase. The viscosity increases drastically following the shear rate decrease, and non-Newtonian viscosity behavior is displayed by the mesophase.
- This article is part of the themed collection: 5th Congress on Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...