Development of biocompatible apatite nanorod-based drug-delivery system with in situ fluorescence imaging capacity
Abstract
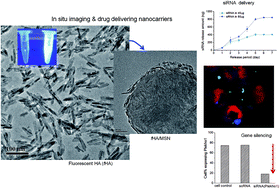
Biocompatible and multifunctional nanocarriers significantly improve the therapeutic and diagnostic efficacy of the cargo molecules. Here we report a novel biocompatible apatite nanocrystal-based delivery system with in situ imaging capacity. A nanorod (40 nm × 10 nm) produced by a citrate-involved sol–gel process expressed a strong blue emission at 427 nm under fluorescence microscopy. The CO2˙− radical impurities present in the apatite crystal lattice account for the fluorescent behavior. The fluorescent nanorods exhibited excellent cell viability (over 90% up to ∼100 μg ml−1 concentration for both osteoblasts and osteoclasts). The nanorods loaded alendronate drug at a high efficiency (10%) and released over 3 days, while enabling in situ fluorescence imaging within the cells. The fluorescent apatite was further hybridized onto the surface of mesoporous nanospheres, aimed at improving the drug-delivery capacity. Small interfering RNA (siRNA) gene, encoding Plekho-1, was effectively loaded to the hybrid nanocarrier, and was sustainably released over 5 days. The siRNA-loaded nanocarrier exhibited excellent osteoblastic uptake (96% efficiency) and gene-silencing effect, suppressing Plekho-1 down to ∼18%, while preserving intracellular fluorescence signals. Taken as a whole, the self-fluorescent nature of apatite nanorods is believed to find potential and versatile applications as biocompatible drug-delivery and in situ imaging systems.

 Please wait while we load your content...
Please wait while we load your content...