Electron-rich graphite-like electrode: stability vs. voltage for Al batteries†
Abstract
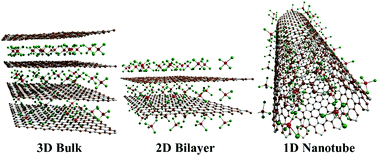
The development of efficient Al batteries is hindered by the major challenge of finding advanced electrode materials, which can deliver higher voltage and storage capacities with ultra-fast charge/discharge rates. Herein, the first principle calculations are used to comparatively study the cathode applicabilities of 3D C3N bulk, 2D C3N bilayer and 1D C3N nanotubes for Al batteries and to investigate the effect of the dimensions of the cathode on the electrochemical properties of the battery. We observe that all three phases of C3N behave in a similar way to those of the graphite counterpart by initiating the charge transfer from the C3N system to intercalated AlCl4. However, an improved diffusivity and storage capacity are obtained for the 1D C3N nanotubes and 2D C3N bilayer as compared to those for the 3D C3N bulk phase in the Al battery. A detailed discussion of stability vs. voltage for the AlCl4 intercalated systems reveals the fact that the electron-donating ability of the C3N system, when compared to that of graphite, results in stronger binding between AlCl4 and the C3N system, which results in a lower net voltage in the Al battery. In this regard, a 1D electron-deficient system with adequate stability towards AlCl4 intercalation can be a superior choice to obtain high voltage in Al batteries when compared to that obtained by using graphite. We believe that our present study will be helpful in understanding the working mechanism of Al batteries and the development of high-voltage Al battery electrodes with adequate stability.


 Please wait while we load your content...
Please wait while we load your content...