Nanoporous hexagonal TiO2 superstructure as a multifunctional material for energy conversion and storage†
Abstract
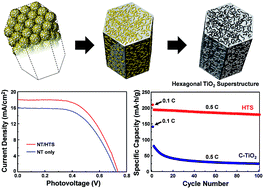
Tremendous efforts have been devoted for the development of rationally designed titanium dioxide (TiO2) nanostructures, because structural characteristics such as morphology, porosity, size, and crystal phase significantly affect the physical properties of TiO2. Despite the significant advances made in synthesis strategies over the past few decades, designing an innovative TiO2 nanostructure that overcomes the limitations that TiO2 encounters in various applications remains a great challenge. In this study, we demonstrate the synthesis of a hierarchically nanostructured TiO2 having a higher symmetry (hexagonal structure) than its own constituent tectons. Anatase TiO2 possesses only a primitive tetragonal unit cell, and there is no hexagonal symmetry axis. Thus, a hexagonal TiO2 superstructure is hardly obtainable without the multiple twinning of primitive units, which rarely occurs under conventional reaction conditions. Our exotic TiO2 nanostructure is synthesized by the calcination of a novel TiO2 precursor that is formed by a simple precipitation reaction and is utilized as an electrode material in energy conversion and storage devices. Due to the unique physical properties offered by this morphology, nanoporous hexagonal TiO2 is superior to conventionally used TiO2 in these applications, highlighting the benefits as an advanced, multifunctional electrode material.

 Please wait while we load your content...
Please wait while we load your content...