Highly selective Fe3+ sensing and proton conduction in a water-stable sulfonate–carboxylate Tb–organic-framework†
Abstract
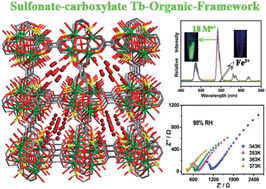
A new 3D porous terbium–organic framework {[Tb4(OH)4(DSOA)2(H2O)8]·(H2O)8}n (Tb-DSOA) has been successfully assembled by Tb3+ ions and a sulfonate–carboxylate linker disodium-2,2′-disulfonate-4,4′-oxydibenzoic acid (Na2H2DSOA). In this metal–organic framework (MOF), tetranuclear terbium clusters can be sensitized by the organic linker to generate the characteristic photoluminescence of TbIII ions. Noncoordinated sulfonate oxygen atoms functionalize its channels, which act as basic sites leading to highly selective Fe3+ ion sensing by luminescence quenching, and also act as hopping sites for proton transfer, resulting in a proton conductivity of 1.66 × 10−4 S cm−1 at 98% RH. Exceptional water-stability makes this MOF compatible for these applications in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...