Understanding the defect chemistry of alkali metal strontium silicate solid solutions: insights from experiment and theory†
Abstract
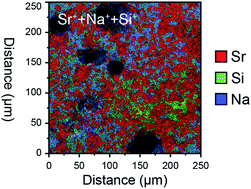
Recent reports of remarkably high oxide ion conduction in a new family of strontium silicates have been challenged. It has recently been demonstrated that, in the nominally potassium substituted strontium germanium silicate material, the dominant charge carrier was not the oxygen ion, and furthermore that the material was not single phase (R. D. Bayliss et. al., Energy Environ. Sci., 2014, DOI: 10.1039/c4ee00734d). In this work we re-investigate the sodium-doped strontium silicate material that was reported to exhibit the highest oxide ion conductivity in the solid solution, nominally Sr0.55Na0.45SiO2.775. The results show lower levels of total conductivity than previously reported and sub-micron elemental mapping demonstrates, in a similar manner to that reported for the Sr0.8K0.2Si0.5Ge0.5O2.9 composition, an inhomogeneous chemical distribution correlating with a multiphase material. It is also shown that the conductivity is not related to protonic mobility. A density functional theory computational approach provides a theoretical justification for these new results, related to the high energetic costs associated with oxygen vacancy formation.

 Please wait while we load your content...
Please wait while we load your content...