A low dimensional composite of hexagonal lithium manganese borate (LiMnBO3), a cathode material for Li-ion batteries†‡
Abstract
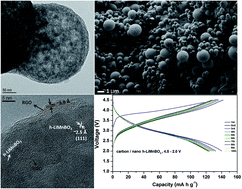
The ultrasonic nebulized spray pyrolysis technique has been applied to synthesize amorphous nanospheres, which are further transformed into nano h-LiMnBO3 with an average crystallite size of ∼14 nm. A composite electrode of nano h-LiMnBO3 with reduced graphite oxide and amorphous carbon delivers a high first discharge capacity of 140 mA h g−1 at C/15 rate within 4.5–2.0 V and retains a discharge capacity of 110 mA h g−1 at the 25th cycle. The dissolution of Mn into the electrolyte and the instability of the highly delithiated phases during cycling are suggested as the reasons, which limit the cycling stability of h-LiMnBO3. An improved cycling stability at higher capacities is expected via the combination of the particle size reduction, conductive network formation and the metal site doping strategies.

 Please wait while we load your content...
Please wait while we load your content...