Photochemical synthesis of bimetallic and anisotropic Au-containing nanoparticles using a one-step protocol†
Abstract
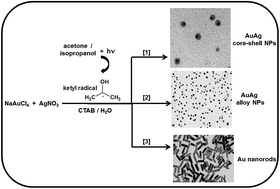
The production of AuAg alloys, AuAg core–shell nanoparticles, and short Au nanorods using ketyl radicals was investigated. The photochemical pair acetone/isopropanol was used as a source of reducing ketyl radicals. The process was optimized in relation to the targeted nanoparticle morphology. For every case, a simple methodology (a 1-step process) was designed aiming at the production of a particular morphology. By the simple adjustment of reagent concentrations and light intensity, the method affords nanoparticles of various compositions with a specific morphology in quantitative yield (either AuAg core–shells, or AuAg alloy nanoparticles, or Au nanorods). In addition, the plasmon properties of the colloidal systems were studied using UV spectroscopy. The mechanism of formation of each nanoparticulate system is discussed giving special attention to the factors responsible for shape and size. The role of acetone in the photochemical preparation of nanorods is further clarified. Transmission electron imaging and X-ray photoelectron spectroscopy analysis were used to characterize the colloidal nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...