A new aspect of Chevrel compounds as positive electrodes for magnesium batteries†
Abstract
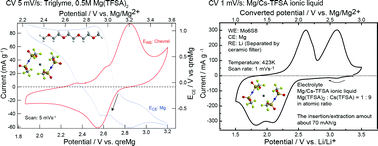
Chevrel compounds are regarded as potential positive-electrode materials for magnesium rechargeable batteries, but their redox potential is only about 1.2 V vs. Mg/Mg2+. In this work, we show logically and experimentally that the redox potential of Chevrel compounds can be as high as about 2–3 V vs. Mg/Mg2+. A crucial basis for this is that Cu cations can be extracted from CuxMo6S8 at around 1.2–1.6 V vs. Mg/Mg2+ in the conventional electrolyte (Grignard-reagent/tetrahydrofuran) while the anodic dissolution of Cu metal can occur above about 1.7 V vs. Mg/Mg2+ in the same electrolyte, which means that the chemical potential of Cu in Chevrel compounds is higher than that in pure Cu metal. This thermodynamic conflict inevitably compels us to consider a certain interaction with the solvent, rather than simple deintercalation from the compound, which is discussed throughout the paper. With the use of large-molecule solvents or ionic liquids, we have observed an intriguing relaxation phenomenon, where the cations move to find more stable sites, which directly indicates that the Chevrel compounds have several sites for cations.

 Please wait while we load your content...
Please wait while we load your content...