Li- and Na-reduction products of meso-Co3O4 form high-rate, stably cycling battery anode materials†
Abstract
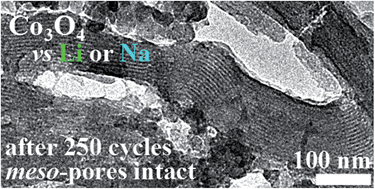
High surface area (367 m2 g−1) meso-porous Co3O4 was investigated as the precursor of the anode material for lithium and also sodium ion batteries. Co3O4 is considered a potential anode material due to its theoretical capacity of 890 mA h g−1, over twice that of graphite. This comparatively higher capacity can be safely charged at rapid rates owing to a relatively high Li-insertion potentials, but, consequently, the discharged energy is yielded at an average potential near 2 V vs. Li/Li+, with full Li-extraction achieved over a continuum of potentials up to 3 V. The products of the lithium reduction of Co3O4 cycle stably from 0.01–3.0 V vs. Li/Li+ with 600–900 mA h g−1 capacity retention at C rates from 1–5; the products of its sodium reduction cycle stably from 0.01–3.0 V vs. Na/Na+ at C-rates up to 1 C with a lower 150–400 mA h g−1 capacity retention owing to greater ionic impedance. TEM, SAED and XRD were used to examine the cycled material and the stable performance is attributed to finding that the mesoporous structure is retained. Evaluation of five electrolyte formulations testing EC, FEC and Cl-EC showed that the stable meso-porous structure was best cycled with 5% FEC in EC:DEC at high charge/discharge rates, retaining 77% of its initial capacity at 5 C in a rate test. Comparison of the AC impedance spectra and of the XPS of the SEIs formed in the presence and in the absence of 5 vol% FEC shows that the SEI formed in the presence of FEC contains lithium fluoride and its carbonate layer is thinner than that formed in its absence, resulting in lesser impedance to Li migration through the SEI and facile ion de-solvation, improving the cycling performance. In cycling stability tests with EC:DEC, irregular cycling behaviour attributable to abrupt rises in cell resistance was regularly observed after testing over a few hundred cycles. Long-term cycling irregularities are inhibited by halogenated solvents and completely eliminated by adding fluoroethylene carbonate (FEC).

 Please wait while we load your content...
Please wait while we load your content...