Acetoacetanilide-functionalized Fe3O4 nanoparticles for selective and cyclic removal of Pb2+ ions from different charged wastewaters†
Abstract
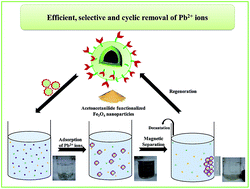
Efficient, selective and reusable acetoacetanilide-functionalized Fe3O4 nanoparticles were developed for the first time for the removal of Pb2+ ions. A comprehensive characterization of the functionalized nanoparticles, at different levels of synthesis, was carried out by TEM, EDS, XRD, SEM, FT-IR and VSM. The adsorption equilibrium obeyed the Langmuir isotherm model with a maximum enrichment capacity of 392.2 mg g−1 at 318 K. Pb2+ ions showed quick removal, and the adsorption rate followed pseudo-second-order kinetics. In addition, isotherm and kinetic studies suggested that the adsorption process is controlled by chemical adsorption, involving the complexation of metal ions with the functional groups present on the surface of the functionalized nanoparticles. The thermodynamic analysis revealed that the analyte adsorption is spontaneous, endothermic and energetically driven in nature. Because the superparamagnetism of the Fe3O4 nanoparticles as the magnetic core and silica coat serve as a protective shell, the adsorbent was easily separated and effectively recycled without significant deterioration in its original performance for at least 10 continuous usages. Furthermore, the proposed environmentally benign analytical process was successfully applied for the selective recovery of Pb2+ ions from different charged wastewaters.

 Please wait while we load your content...
Please wait while we load your content...