Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method†
Abstract
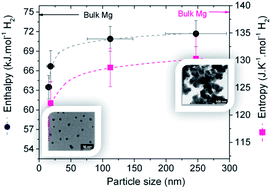
Magnesium nanoparticles have been synthesized by reducing di-n-butylmagnesium with lithium in the presence of naphthalene as an electron carrier. The reactants' ratio significantly influenced the particle size leading to some control of the nucleation and growth process with magnesium particles as small as 8 nm produced at high lithium/naphthalene ratios. Studies of hydrogen storage properties showed that these materials were capable of hydrogen uptake at low temperatures (<150 °C) with kinetics dependent to some extent on the particle size. More remarkably, thermodynamics were found to significantly deviate from that of bulk MgH2 with a significant decrease of both enthalpy (to 63.5 ± 1.8 kJ mol−1 H2) and entropy (to 118.4 ± 3.1 J K−1 mol−1 H2) for particle sizes below 25 nm. These effects are much larger than previously thought and demonstrate that even relatively large isolated nanoparticles can undergo significant thermodynamic alterations.

 Please wait while we load your content...
Please wait while we load your content...