Enhanced electrochemical performances of Li-rich layered oxides by surface modification with reduced graphene oxide/AlPO4 hybrid coating†
Abstract
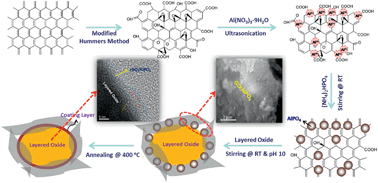
The electrochemical performance of lithium-rich layered oxide cathode materials coated with reduced graphene oxide (rGO)/AlPO4 hybrid coatings has been investigated. The amount of graphene was varied from 0.5 to 4.0 wt%, while AlPO4 was maintained at 2.0 wt%. The structure and morphology of the samples have been investigated using X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and X-ray photoelectron spectroscopy. Compared to the pristine cathode, the rGO/AlPO4-coated cathodes exhibit higher discharge capacity with lower irreversible capacity loss, better cyclability, and higher rate capability. Specifically, the rGO (0.5 wt%)/AlPO4 (2.0 wt%)- and rGO (1.0 wt%)/AlPO4 (2.0 wt%)-coated samples exhibit high discharge capacities of 235 mA h g−1 and 240 mA h g−1, respectively, at C/10 rate with capacity retentions of 94% and 95%, respectively, at 100 cycles. Moreover, the rGO/AlPO4-coated cathodes show better cyclability than the pristine cathode at an elevated temperature (55 °C). The superior electrochemical performances of the rGO/AlPO4-coated electrodes could be attributed to the decrease in the Li content in the solid-electrolyte interphase (SEI) layer and facilitation of faster charge transfer as suggested by XPS and electrochemical impedance spectroscopy (EIS).

 Please wait while we load your content...
Please wait while we load your content...