Fabrication and performance of a Mn–La metal composite for remarkable decontamination of fluoride
Abstract
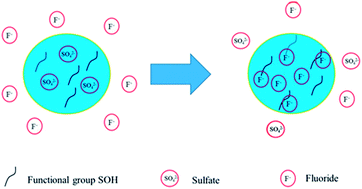
Fluoride contamination has become a great issue of concern in recent years as it could cause a high risk to human health through water consumption. In this study, a new manganese–lanthanum bimetal composite was developed by a co-precipitation method. The adsorption was highly pH dependent, and the optimal adsorption was obtained at pH 5.0. The Langmuir equation better fitted the experimental data of the adsorption isotherm. The maximum adsorption capacity of 292.9 mg g−1 was achieved at the optimal pH, much higher than most currently reported sorbents. The adsorption occurred rapidly in the first 1 h; the adsorption equilibrium was reached within 8 h. The presence of chloride, sulfate, carbonate and humic acid had an insignificant influence on the fluoride uptake; however, phosphate greatly hindered the removal. The adsorption reactions due to the presence of weak acid functional groups and the ion exchange reaction between sulfate and fluoride could be responsible for the removal of fluoride. The adsorption history was described by the intraparticle diffusion model.

 Please wait while we load your content...
Please wait while we load your content...