High voltage sulphate cathodes Li2M(SO4)2 (M = Fe, Mn, Co): atomic-scale studies of lithium diffusion, surfaces and voltage trends†
Abstract
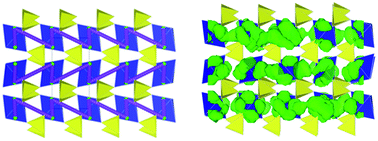
The search for high voltage cathodes for lithium-ion batteries has led to recent interest in the monoclinic Li2Fe(SO4)2 material which has a voltage of 3.83 V vs. lithium, the highest recorded for a fluorine-free iron-based compound. Here we investigate the defect, surface and lithium migration properties of the Li2M(SO4)2 (M = Fe, Mn, Co) materials using combined atomistic modelling and density functional theory (DFT) techniques. All intrinsic defect types including Li/M antisite disorder are found to be of high energy, suggesting insignificant concentrations. Low activation energies are found for lithium migration along the a-axis channels giving rise to long-range 1D diffusion, which are supported by molecular dynamics (MD) simulations. For the crystal morphology a significant surface area is exposed to these 1D diffusion channels, which would allow facile Li insertion and extraction. Using DFT simulations we reproduce the high voltage of the Li2Fe(SO4)2 material in accord with electrochemical data and also examine local structural distortions on lithium extraction.

 Please wait while we load your content...
Please wait while we load your content...