Site-exchange of Li and M ions in silicate cathode materials Li2MSiO4 (M = Mn, Fe, Co and Ni): DFT calculations
Abstract
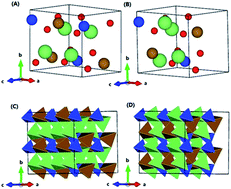
First principles calculations have been used to investigate the occurrence of site-exchange of Li and M ions and the effect of the site-exchange on Li extraction of silicate cathode materials Li2MSiO4 (M = Mn, Fe, Co and Ni). Total energy calculations suggest that Li (in the 4b site) and M (in the 2a site) ions become site-exchanged upon delithiation. This structural arrangement leads to significant cell expansion as two Li ions are extracted. Elastic property calculations indicate that the ductility of the fully delithiated MSiO4 is impaired, which is likely to induce structural collapse and rapid capacity attenuation during the charge–discharge cycles. Restraining the site-exchange or improving the ductility may be an effective way of developing high performance Li2MSiO4 for Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...