The interaction of H3PO4 and steam with PBI and TPS polymeric membranes. A TGA and Raman study
Abstract
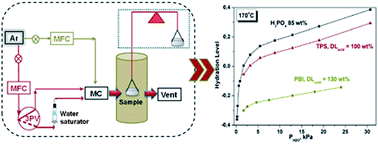
Thermogravimetric analysis (TGA) and Raman spectroscopic experiments were carried out in order to study the interaction of H3PO4 and steam with the chemical structure of polybenzimidazole (PBI) and pyridine bearing aromatic polyethers (TPS). The interaction of the polymers with H3PO4 causes a blue Raman shift on the imidazole and the pyridine bands, which is larger for the imidazole ring. It has been found that one H3PO4 molecule is needed to neutralize one imidazole group, while ca. five H3PO4 molecules are necessary for full protonation of the pyridine group. This observation is reflected on the hydration procedure of the imbibed membranes at high temperatures (150–170 °C) as this was studied by TGA. At low acid doping levels (130–150 wt%) the hydrolysis of pyrophosphoric acid into ortho-phosphoric acid is not reversible in the case of PBI, thus indicating that the chemical structure influences the chemical equilibrium of the hydrolysis reaction. The evaporation rate of H3PO4 increases with increasing steam partial pressure and is lower in the case of PBI than in TPS. Nevertheless in all cases the evaporation rate is by a factor of two lower than that of the pure phosphoric acid under the same steam partial pressure.

 Please wait while we load your content...
Please wait while we load your content...