Structural tailoring of hydrogen-bonded poly(acrylic acid)/poly(ethylene oxide) multilayer thin films for reduced gas permeability†
Abstract
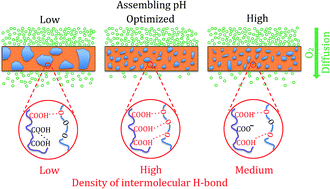
Hydrogen bonded poly(acrylic acid) (PAA)/poly(ethylene oxide) (PEO) layer-by-layer assemblies are highly elastomeric, but more permeable than ionically bonded thin films. In order to expand the use of hydrogen-bonded assemblies to applications that require a better gas barrier, the effect of assembling pH on the oxygen permeability of PAA/PEO multilayer thin films was investigated. Altering the assembling pH leads to significant changes in phase morphology and bonding. The amount of intermolecular hydrogen bonding between PAA and PEO is found to increase with increasing pH due to reduction of COOH dimers between PAA chains. This improved bonding leads to smaller PEO domains and lower gas permeability. Further increasing the pH beyond 2.75 results in higher oxygen permeability due to partial deprotonation of PAA. By setting the assembling pH at 2.75, the negative impacts of COOH dimer formation and PAA ionization on intermolecular hydrogen bonding can be minimized, leading to a 50% reduction in the oxygen permeability of the PAA/PEO thin film. A 20 bilayer coating reduces the oxygen transmission rate of a 1.58 mm natural rubber substrate by 20×. These unique nanocoatings provide the opportunity to impart a gas barrier to elastomeric substrates without altering their mechanical behavior.


 Please wait while we load your content...
Please wait while we load your content...