Micro-topography influences blood platelet spreading†
Abstract
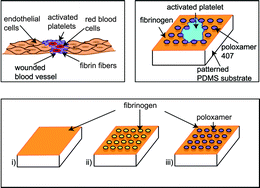
Injuries in blood vessels are accompanied by disrupted endothelial cell layers. Missing or destroyed endothelial cells lead to rough, structured surfaces on the micrometer scale. The first cells to arrive at the site of injury and to cover the wound are platelets, which subsequently drive blood clot formation. Therefore, investigating the interactions of platelets with structured surfaces is essential for the understanding of blood clotting. Here, we study the effects of underlying topography on platelet spreading using microstructured model substrates with varying area fractions of protein coating. We thereby distinguish the effects of (physical) topography and of (biochemical) protein availability. By analyzing the cell area and morphology, we find that the extent of protrusion formation – but not the total spread area – is determined by the area fractions of coating. The extent of filopodia formation is influenced by the availability of binding sites and the reaction of cells to the substrate's topography. The cells react to the structured substrate by avoiding topographic holes at the cell periphery and thus adapting their outer shape. This finding leads us to the conclusion that both chemically blocked and fibrinogen-coated holes represent “energetic obstacles” to the cells. Thus, the shape of the cell is governed by the interplay between spreading to an optimized area and adaption to the substrate topography.
- This article is part of the themed collection: Proteins, cells, and tissues in patterned environments

 Please wait while we load your content...
Please wait while we load your content...