Free energy change for insertion of charged, monolayer-protected nanoparticles into lipid bilayers†
Abstract
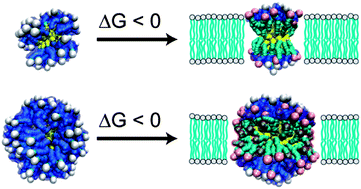
Charged, monolayer-protected gold nanoparticles (AuNPs) with core diameters smaller than 10 nm have recently emerged as a prominent class of nanomaterial for use in targeted drug delivery and biosensing. In particular, recent experimental studies showed that AuNPs protected by a binary mixture of purely hydrophobic and anionic, end-functionalized alkanethiol ligands were able to spontaneously penetrate through cell membranes via a non-endocytic, non-disruptive mechanism. The critical step in the penetration process is a fusion step during which the AuNPs insert into the hydrophobic core of the bilayer. This fusion step is driven by hydrophobic forces as inserted AuNPs minimize their exposed hydrophobic surface area and thereby lower their free energy compared to particles in the bulk. Here, we explore the effect of the large parameter space of composition, size, ligand length, morphology, and hydrophobicity strength on the change in the free energy upon insertion. Using a newly developed implicit bilayer, implicit solvent simulation model, our work shows that there is a size cutoff for insertion that has a strong dependence on surface composition and ligand chemistry. Our results agree well with previous experimental findings for a particular value of the hydrophobicity strength. This work provides physical insight that may be used to both understand the insertion of AuNPs into bilayers and guide the design of monolayers to either encourage or inhibit insertion.

 Please wait while we load your content...
Please wait while we load your content...