Bioorthogonal prodrug activation driven by a strain-promoted 1,3-dipolar cycloaddition†
Abstract
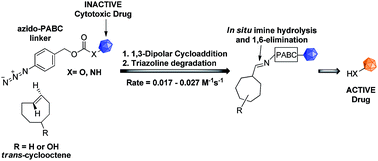
Due to the formation of hydrolysis-susceptible adducts, the 1,3-dipolar cycloaddition between an azide and strained trans-cyclooctene (TCO) has been disregarded in the field of bioorthogonal chemistry. We report a method which uses the instability of the adducts to our advantage in a prodrug activation strategy. The reaction of trans-cyclooctenol (TCO-OH) with a model prodrug resulted in a rapid 1,3-dipolar cycloaddition with second-order rates of 0.017 M−1 s−1 and 0.027 M−1 s−1 for the equatorial and axial isomers, respectively, resulting in release of the active compound. 1H NMR studies showed that activation proceeded via a triazoline and imine, both of which are rapidly hydrolyzed to release the model drug. Cytotoxicity of a doxorubicin prodrug was restored in vitro upon activation with TCO-OH, while with cis-cyclooctenol (CCO-OH) no activation was observed. The data also demonstrates the potential of this reaction in organic synthesis as a mild orthogonal protecting group strategy for amino and hydroxyl groups.

 Please wait while we load your content...
Please wait while we load your content...