Merging of the photocatalysis and copper catalysis in metal–organic frameworks for oxidative C–C bond formation†
Abstract
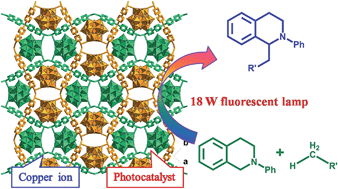
The direct formation of new C–C bonds through photocatalytic oxidative coupling from low reactive sp3 C–H bonds using environmentally benign and cheap oxygen as oxidant is an important area in sustainable chemistry. By incorporating the photoredox catalyst [SiW11O39Ru(H2O)]5− into the pores of Cu-based metal–organic frameworks, a new approach for merging Cu-catalysis/Ru-photocatalysis within one single MOF was achieved. The direct CuII–O–W(Ru) bridges made the two metal catalyses being synergetic, enabling the application on the catalysis of the oxidative coupling C–C bond formation from acetophenones and N-phenyl-tetrahydroisoquinoline with excellent conversion and size-selectivity. The method takes advantage of visible light photoredox catalysis to generate iminium ion intermediate from N-phenyl-tetrahydroisoquinoline under mild conditions and the easy combination with Cu-catalyzed activation of nucleophiles. Control catalytic experiments using similar Cu-based sheets but with the photoredox catalytic anions embedded was also investigated for comparison.

 Please wait while we load your content...
Please wait while we load your content...