The complete conformational free energy landscape of β-xylose reveals a two-fold catalytic itinerary for β-xylanases†
Abstract
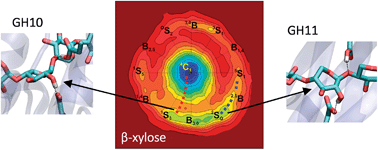
Unraveling the conformational catalytic itinerary of glycoside hydrolases (GHs) is a growing topic of interest in glycobiology, with major impact in the design of GH inhibitors. β-xylanases are responsible for the hydrolysis of glycosidic bonds in β-xylans, a group of hemicelluloses of high biotechnological interest that are found in plant cell walls. The precise conformations followed by the substrate during catalysis in β-xylanases have not been unambiguously resolved, with three different pathways being proposed from structural analyses. In this work, we compute the conformational free energy landscape (FEL) of β-xylose to predict the most likely catalytic itineraries followed by β-xylanases. The calculations are performed by means of ab initio metadynamics, using the Cremer–Pople puckering coordinates as collective variables. The computed FEL supports only two of the previously proposed itineraries, 2SO → [2,5B]ǂ → 5S1 and 1S3 → [4H3]ǂ → 4C1, which clearly appear in low energy regions of the FEL. Consistently, 2SO and 1S3 are conformations preactivated for catalysis in terms of free energy/anomeric charge and bond distances. The results however exclude the OE → [OS2]ǂ → B2,5 itinerary that has been recently proposed for a family 11 xylanase. Classical and ab initio QM/MM molecular dynamics simulations reveal that, in this case, the observed OE conformation has been enforced by enzyme mutation. These results add a word of caution on using modified enzymes to inform on catalytic conformational itineraries of glycoside hydrolases.

 Please wait while we load your content...
Please wait while we load your content...