Anionic polymerisation of phosphaalkenes bearing polyaromatic chromophores: phosphine polymers showing “turn-on” emission selectively with peroxide†
Abstract
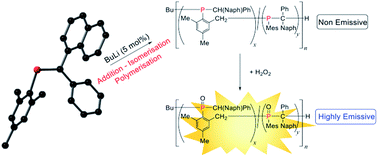
Three new phosphaalkenes bearing C-aryl chromophores, MesP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh(Ar) (1a: Ar = 1-naphthyl, 1b: 9-phenanthryl, 1c: 5-dibenzosuberenyl; Mes = 2,4,6-trimethylphenyl), are reported. Each phosphaalkene was characterised by multinuclear NMR spectroscopy (1H, 13C, 31P), X-ray crystallography, mass spectrometry, UV/Visible spectroscopy and elemental microanalysis (1b and 1c). Monomers 1a and 1b were successfully polymerised using anionic methods of initiation (n-BuLi: 5 and 1.5 mol%, respectively) to afford poly(methylenephosphine)s (PMPs: 2a: Mn = 15 100 Da, PDI = 1.14; 2b: Mn = 17 500 Da; PDI = 1.39). Detailed NMR spectroscopic analysis of polymer 2a revealed that its microstructure is primarily composed of [P(CHPhNaph)–(4,6-Me2C6H2)–2-CH2]n units rather than the expected [P(Mes)–CPh2]n. Functional polymer 2a was oxidised to the phosphine oxide 2a·O or phosphine sulfide 2a·S, and coordinated to borane to afford 2a·BH3 or gold(I) to afford 2a·AuCl. Significantly, 2a shows “turn-on” emission properties selectively as its oxide 2a·O (λex = 286 nm; λem = 342 nm) which is prepared by treatment with hydrogen peroxide.
CPh(Ar) (1a: Ar = 1-naphthyl, 1b: 9-phenanthryl, 1c: 5-dibenzosuberenyl; Mes = 2,4,6-trimethylphenyl), are reported. Each phosphaalkene was characterised by multinuclear NMR spectroscopy (1H, 13C, 31P), X-ray crystallography, mass spectrometry, UV/Visible spectroscopy and elemental microanalysis (1b and 1c). Monomers 1a and 1b were successfully polymerised using anionic methods of initiation (n-BuLi: 5 and 1.5 mol%, respectively) to afford poly(methylenephosphine)s (PMPs: 2a: Mn = 15 100 Da, PDI = 1.14; 2b: Mn = 17 500 Da; PDI = 1.39). Detailed NMR spectroscopic analysis of polymer 2a revealed that its microstructure is primarily composed of [P(CHPhNaph)–(4,6-Me2C6H2)–2-CH2]n units rather than the expected [P(Mes)–CPh2]n. Functional polymer 2a was oxidised to the phosphine oxide 2a·O or phosphine sulfide 2a·S, and coordinated to borane to afford 2a·BH3 or gold(I) to afford 2a·AuCl. Significantly, 2a shows “turn-on” emission properties selectively as its oxide 2a·O (λex = 286 nm; λem = 342 nm) which is prepared by treatment with hydrogen peroxide.

 Please wait while we load your content...
Please wait while we load your content...