Dark to light! A new strategy for large Stokes shift dyes: coupling of a dark donor with tunable high quantum yield acceptors†
Abstract
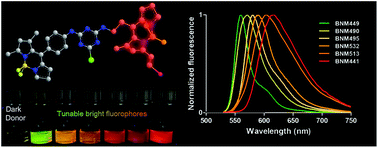
A new strategy for constructing large Stokes shift dyes by coupling a low quantum yield (less than 1%) BODIPY donor (BDN) with tunable high quantum yield BODIPY acceptors (BDM) has been explored to synthesize a set of novel Dark Resonance Energy Transfer (DRET) dyes, named BNM. The low quantum yield of the donor is ascribed to the intramolecular rotation of the phenyl rings, which has been proven by controlling the viscosity and temperature of the solvent. However, upon excitation of BNM compounds at the donor absorption wavelength, tunable emissions from 560 nm to 617 nm were obtained, with a high quantum yield of up to 0.75. Ultrafast dynamic studies demonstrated that the absorbed energy was transferred to the acceptor (BDM) with a high energy transfer rate, before being quenched by non-radiative intramolecular rotations. Using a dark donor makes it possible to avoid fluorescence leaks from donor emission. This is a new set of RET dyes that can be excited by a low quantum yield donor to emit a tunable wide range of high fluorescence emission.

 Please wait while we load your content...
Please wait while we load your content...