Submillisecond-lived photoinduced charge separation in a fully conjugated phthalocyanine–perylenebenzimidazole dyad†
Abstract
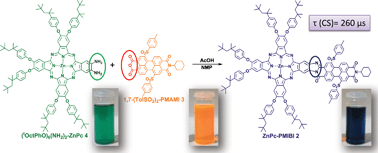
A fully electronically conjugated phthalocyanine–perylenemonoimidebenzimidazole system, ZnPc–PMIBI 2, where the conjugation goes through the imide position of the perylene has been synthesized. The preparation was made possible by the condensation of a new unsymmetrically substituted diaminophthalocyanine, ZnPc(NH2)2, with a perylene monoanhydride monoimide. Both the experimental and the computational (DFT) results indicate that ZnPc–PMIBI exhibits significant intramolecular electronic interactions. The lifetime of the charge-separated (CS) state was extended to 0.26 ms, corresponding to the longest value ever reported for a covalent phthalocyanine–peryleneimide system in solution, and is attributed to the synergy of an extremely low CS energy, lower than the triplet energy of each chromophore, together with the coupling between both units, allowing fast charge separation.

 Please wait while we load your content...
Please wait while we load your content...