Uncovering divergent evolution of α/β-hydrolases: a surprising residue substitution needed to convert Hevea brasiliensis hydroxynitrile lyase into an esterase†
Abstract
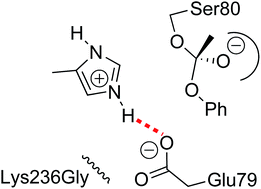
Hevea brasiliensis hydroxynitrile lyase (HbHNL) and salicylic acid binding protein 2 (SABP2, an esterase) share 45% amino acid sequence identity, the same protein fold, and even the same catalytic triad of Ser-His-Asp. However, they catalyze different reactions: cleavage of hydroxynitriles and hydrolysis of esters, respectively. To understand how other active site differences in the two enzymes enable the same catalytic triad to catalyze different reactions, we substituted amino acid residues in HbHNL with the corresponding residues from SABP2, expecting hydroxynitrile lyase activity to decrease and esterase activity to increase. Previous mechanistic studies and X-ray crystallography suggested that esterase activity requires removal of an active site lysine and threonine from the hydroxynitrile lyase. The Thr11Gly Lys236Gly substitutions in HbHNL reduced hydroxynitrile lyase activity for cleavage of mandelonitrile 100-fold, but increased esterase activity only threefold to kcat ∼0.1 min−1 for hydrolysis of p-nitrophenyl acetate. Adding a third substitution – Glu79His – increased esterase activity more than tenfold to kcat ∼1.6 min−1. The specificity constant (kcat/KM) for this triple substitution variant versus wild type HbHNL shifted more than one million-fold from hydroxynitrile lyase activity (acetone cyanohydrin substrate) to esterase activity (p-nitrophenyl acetate substrate). The contribution of Glu79His to esterase activity was surprising since esterases and lipases contain many different amino acids at this position, including glutamate. Saturation mutagenesis at position 79 showed that 13 of 19 possible amino acid substitutions increased esterase activity, suggesting that removal of glutamate, not addition of histidine, increased esterase activity. Molecular modeling indicates that Glu79 disrupts esterase activity in HbHNL when its negatively charged side chain distorts the orientation of the catalytic histidine. Naturally occurring glutamate at the corresponding location of Candida lipases is uncharged due to other active site differences and does not cause the same distortion. This example of the fine tuning of the same catalytic triad for different types of catalysis by subtle interactions with other active site residues shows how difficult it is to design new catalytic reactions of enzymes.

 Please wait while we load your content...
Please wait while we load your content...