Discovery of a cyclic 6 + 6 hexamer of d-biotin and formaldehyde†
Abstract
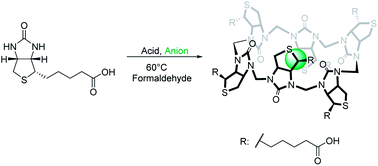
The discovery of receptors using templated synthesis enables the selection of strong receptors from complex mixtures. In this contribution we describe a study of the condensation of D-biotin and formaldehyde in acidic water. We have discovered that halide anions template the formation of a single isomer of a 6 + 6 macrocycle. The macrocycle (biotin[6]uril) is water-soluble, chiral and binds halide anions (iodide, bromide and chloride) with selectivity for iodide in water, and it can be isolated on a gram scale in a one-pot reaction in 63% yield.

 Please wait while we load your content...
Please wait while we load your content...