Experimental and computational studies on the mechanism of the Pd-catalyzed C(sp3)–H γ-arylation of amino acid derivatives assisted by the 2-pyridylsulfonyl group†
Abstract
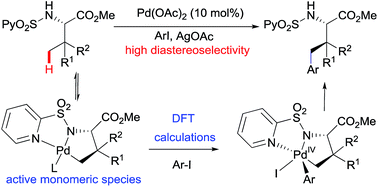
The Pd(OAc)2-catalyzed γ-arylation of amino acid esters bearing a removable N-(2-pyridyl)sulfonyl directing group via C(sp3)–H activation provides a direct method to form functionalized amino acids without racemization at the α-C and with a high degree of stereoselectivity. The present mechanistic studies suggest that the reaction proceeds via a catalytically active monomeric species, and that the C–H activation is reversible and is not always the turnover limiting step. Moreover, theoretical calculations explain the observed stereoselectivity and suggest that the reaction proceeds through a Pd(II)/Pd(IV) mechanism.

 Please wait while we load your content...
Please wait while we load your content...