Cobaltate anion couples terminal dienes with trifluoroacetic anhydride: a direct fluoroacylation of 1,3-dienes†
Abstract
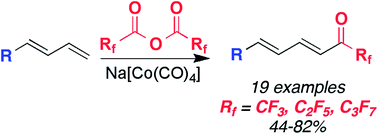
Perfluoroketones are useful products and intermediates in medicinal chemistry. Herein, cobalt-mediated fluoroacylation of 1,3-dienes is described using perfluorinated anhydrides such as TFAA. The reaction is thought to proceed through a fluoroacylcobalt reagent formed in situ. Perfluoroacylation of 1,3 dienes can also be performed to attain longer chain perfluorinated ketones.

 Please wait while we load your content...
Please wait while we load your content...