Inhibition of the 4Fe–4S proteins IspG and IspH: an EPR, ENDOR and HYSCORE investigation†
Abstract
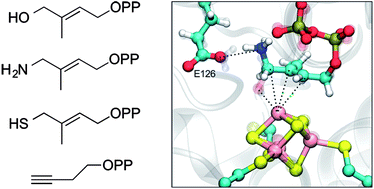
IspG and IspH are proteins that are involved in isoprenoid biosynthesis in most bacteria as well as in malaria parasites and are important drug targets. They contain cubane-type 4Fe–4S clusters that are involved in unusual 2H+/2e− reductions. Here, we report the results of electron paramagnetic resonance spectroscopic investigations of the binding of amino- and thiolo-HMBPP (HMBPP = E-1-hydroxy-2-methyl-but-2-enyl 4-diphosphate) IspH substrate-analog inhibitors to both proteins, as well as the binding of HMBPP and an acetylene diphosphate inhibitor, to IspG. The results show that amino-HMBPP binds to reduced IspH by Fe–C π-bonding with the olefinic carbons interacting with the unique 4th Fe in the 4Fe–4S cluster, quite different to the direct Fe–N ligation seen with the oxidized protein. No such π-complex is observed when amino-HMBPP binds to reduced IspG. No EPR signal is observed with IspH in the presence of dithionite and thiolo-HMBPP, suggesting that the 4Fe–4S cluster is not reduced, consistent with the presence of a 420 nm feature in the absorption spectrum (characteristic of an oxidized cluster). However, with IspG, the EPR spectrum in the presence of dithionite and thiolo-HMBPP is very similar to that seen with HMBPP. The binding of HMBPP to IspG was studied using hyperfine sublevel correlation spectroscopy with 17O and 13C labeled samples: the results rule out direct Fe–O bonding and indicate π-bonding. Finally, the binding to IspG of a potent acetylene diphosphate inhibitor was studied by using electron-nuclear double resonance spectroscopy with 13C labeled ligands: the large hyperfine couplings indicate strong Fe–C π-bonding with the acetylenic group. These results illustrate a remarkable diversity in binding behavior for HMBPP-analog inhibitors, opening up new routes to inhibitor design of interest in the context of anti-bacterial and anti-malarial drug discovery, as well as “cubane-type” metallo-biochemistry, in general.

 Please wait while we load your content...
Please wait while we load your content...