Mechanistic insights into the malonoyl peroxide syn-dihydroxylation of alkenes†
Abstract
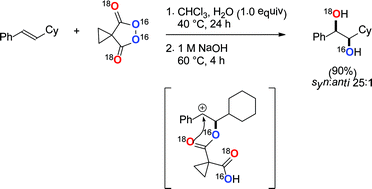
A detailed mechanistic understanding of the malonoyl peroxide mediated dihydroxylation of alkenes is presented. The reaction is first order in both alkene and peroxide with stoichiometric water playing a dual role. An ionic mechanism is proposed and supported by the use of 18O isotopically labelled peroxide, a radical clock probe and DFT calculations. Hammett analysis suggests the reaction proceeds via a discrete carbocation intermediate which is consistent with the stereochemical outcome of the transformation. A subsequent Woodward-type 1,3-dioxolan-2-yl cation has been trapped in situ and the mechanism of hydrolysis defined by isotopic labelling studies. Stable reaction intermediates have been isolated and characterised by X-ray crystallographic analysis and minor competing reaction pathways identified.

 Please wait while we load your content...
Please wait while we load your content...