A kinetically blocked 1,14:11,12-dibenzopentacene: a persistent triplet diradical of a non-Kekulé polycyclic benzenoid hydrocarbon†
Abstract
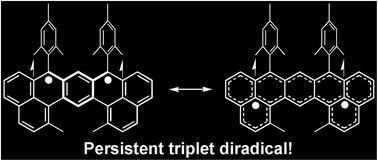
The synthesis of high-spin polycyclic hydrocarbons is very challenging due to their extremely high reactivity. Herein, we report the synthesis and characterization of a kinetically blocked 1,14:11,12-dibenzopentacene, DP-Mes, which represents a rare persistent triplet diradical of a non-Kekulé polycyclic benzenoid hydrocarbon. In contrast to its structural isomer 1,14:7,8-dibenzopentacene (heptazethrene) with a singlet biradical ground state, DP-Mes is a triplet diradical as confirmed by ESR and ESTN measurements and density functional theory calculations. DP-Mes also displays intermolecular antiferromagnetic spin interactions in solution at low temperature.

 Please wait while we load your content...
Please wait while we load your content...