Hydrogen atom abstraction reactions independent of C–H bond dissociation energies of organic substrates in water: significance of oxidant–substrate adduct formation†
Abstract
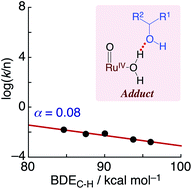
Detailed kinetic studies on the oxidation reactions of organic substrates such as methanol with RuIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O complexes as oxidants, formed electrochemically in water, have been conducted to elucidate the reaction mechanism. The rate constants of the oxidation reactions exhibited saturation behaviours relative to the substrate concentration, regardless of the oxidants and the substrates employed. This indicates the existence of a pre-equilibrium process based on the adduct formation between the RuIV
O complexes as oxidants, formed electrochemically in water, have been conducted to elucidate the reaction mechanism. The rate constants of the oxidation reactions exhibited saturation behaviours relative to the substrate concentration, regardless of the oxidants and the substrates employed. This indicates the existence of a pre-equilibrium process based on the adduct formation between the RuIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxidant and the substrate. Herein, we have experimentally confirmed that the driving force of the adduct formation is the hydrogen bonding between the oxidants and alcohols even in water. In addition, we have investigated the kinetic isotope effects (KIE) on the oxidation reaction using methanol and its deuterated derivatives and as a result observed moderate KIE values for the C–H bond of methanol. We have also revealed the independency of the reaction rates from the bond dissociation enthalpies of the C–H bonds of the substrates. This independency is probably derived from the tightly condensed transition state, whose energy level is strongly controlled by the activation entropy but less sensitive to the activation enthalpy.
O oxidant and the substrate. Herein, we have experimentally confirmed that the driving force of the adduct formation is the hydrogen bonding between the oxidants and alcohols even in water. In addition, we have investigated the kinetic isotope effects (KIE) on the oxidation reaction using methanol and its deuterated derivatives and as a result observed moderate KIE values for the C–H bond of methanol. We have also revealed the independency of the reaction rates from the bond dissociation enthalpies of the C–H bonds of the substrates. This independency is probably derived from the tightly condensed transition state, whose energy level is strongly controlled by the activation entropy but less sensitive to the activation enthalpy.

 Please wait while we load your content...
Please wait while we load your content...