Ruthenium-catalysed Z-selective cross metathesis of allylic-substituted olefins†
Abstract
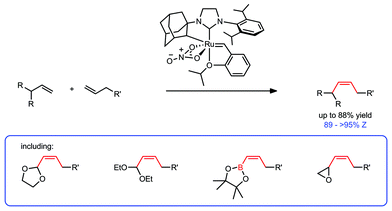
The Z-selective cross metathesis of allylic-substituted olefins is explored with recently developed ruthenium-based metathesis catalysts. The reaction proceeds with excellent stereoselectivity for the Z-isomer (typically >95%) and yields of up to 88% for a variety of allylic substituents. This includes the first synthesis of Z-α,β-unsaturated acetals by cross metathesis and their elaboration to Z-α,β-unsaturated aldehydes. In addition, the reaction is tolerant of a variety of cross partners, varying in functionality and steric profile.

 Please wait while we load your content...
Please wait while we load your content...